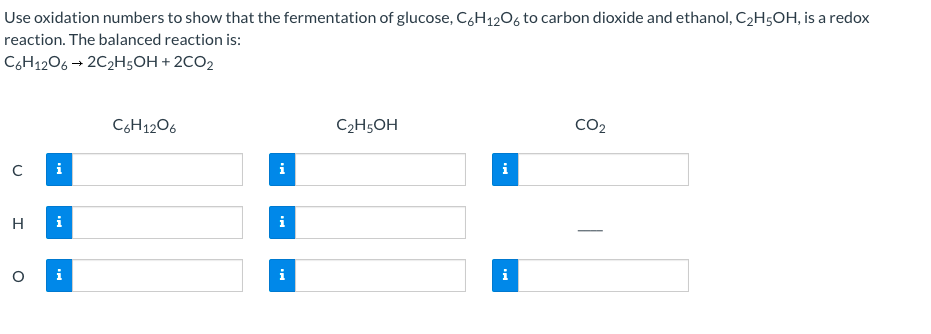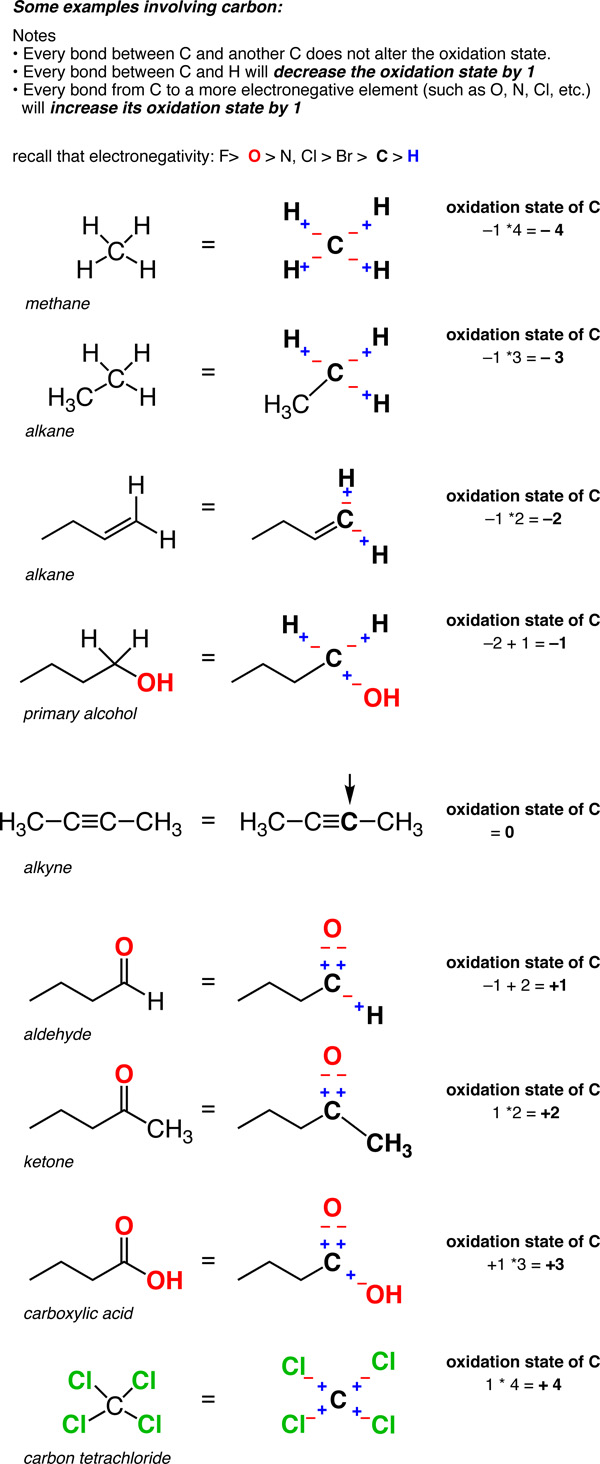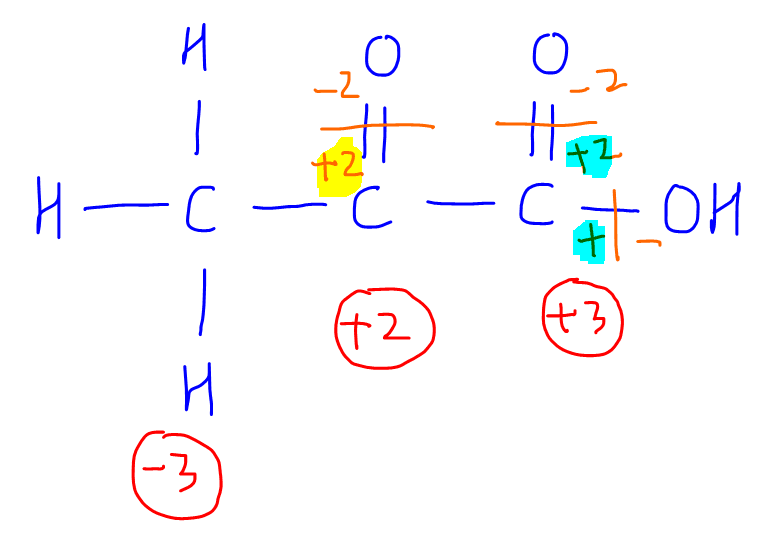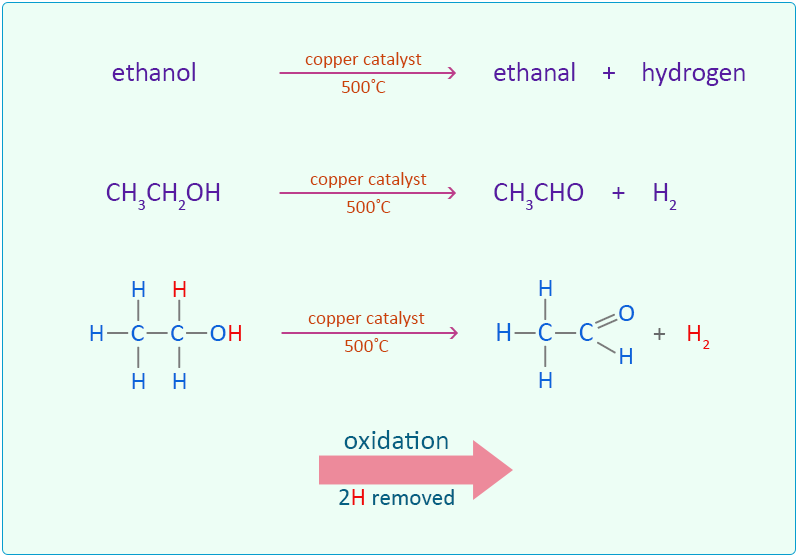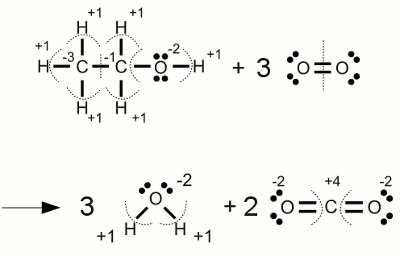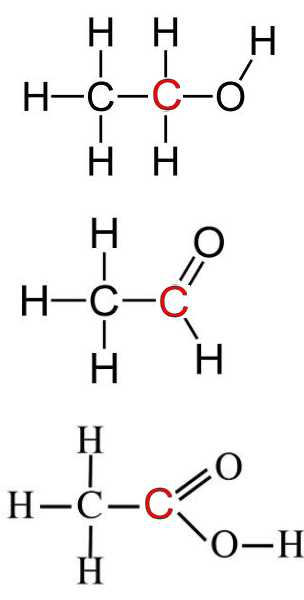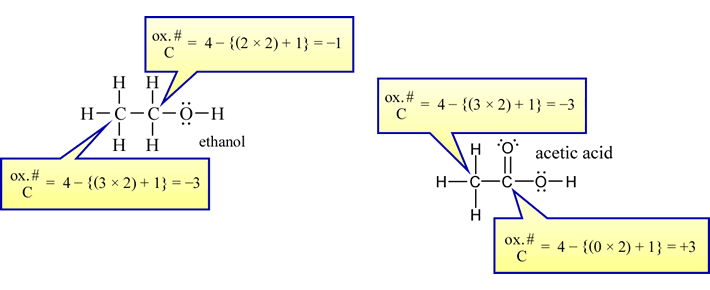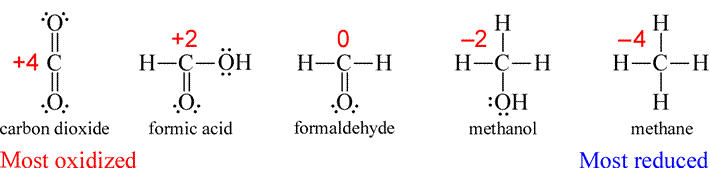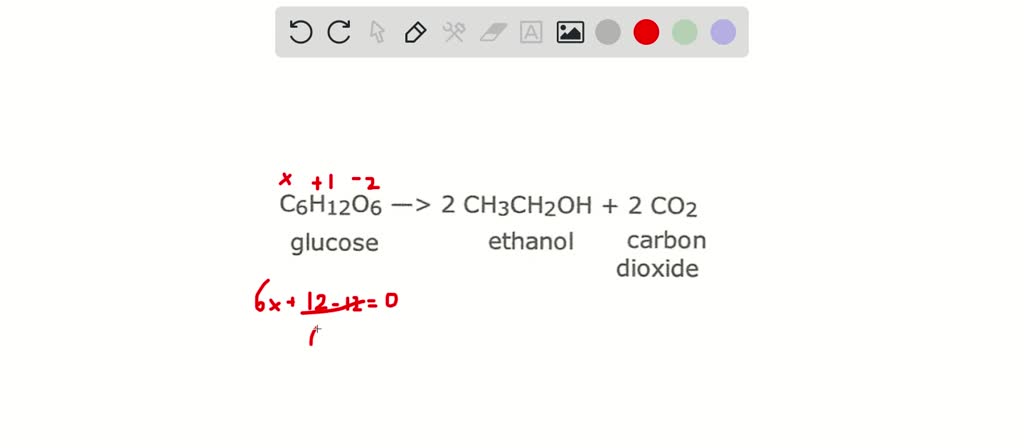
SOLVED:Use oxidation numbers to show that the fermentation of glucose, C6 H12 O6, to carbon dioxide and ethanol, C2 H5 OH, is a redox reaction.

Find the oxidation state of carbon in C2H5OH. oxidation number for c2h5oh @mydocumentary838 - YouTube

electrochemistry - Are there alternative ways to find n or number of e- in problems like the one pictured? - Chemistry Stack Exchange

SOLVED: 35.Wine contains ethanol, CHCHzOH: During the fermentation of wine, care must be taken so that ethanol is not converted to acetic acid, CH3COOH, with the result that the wine is converted

Tafel plot of Pd 86 Sn 14 /C for ethanol oxidation in 0.5 M KOH with... | Download Scientific Diagram

Which one has the highest oxidation state for two-carbon compound? a. Ethane. b. Ethanol. c. Acetaldehyde. d. Acetic acid. | Homework.Study.com

What is the oxidation Number of each carbon in CH3CH2OH? Rules of assigning oxidation numbers. - YouTube

Which one has the highest oxidation state for two-carbon compound? a. Ethane. b. Ethanol. c. Acetaldehyde. d. Acetic acid. | Homework.Study.com