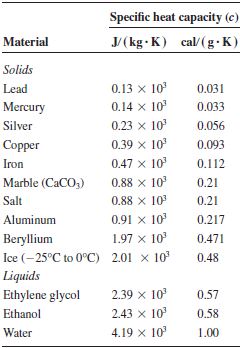
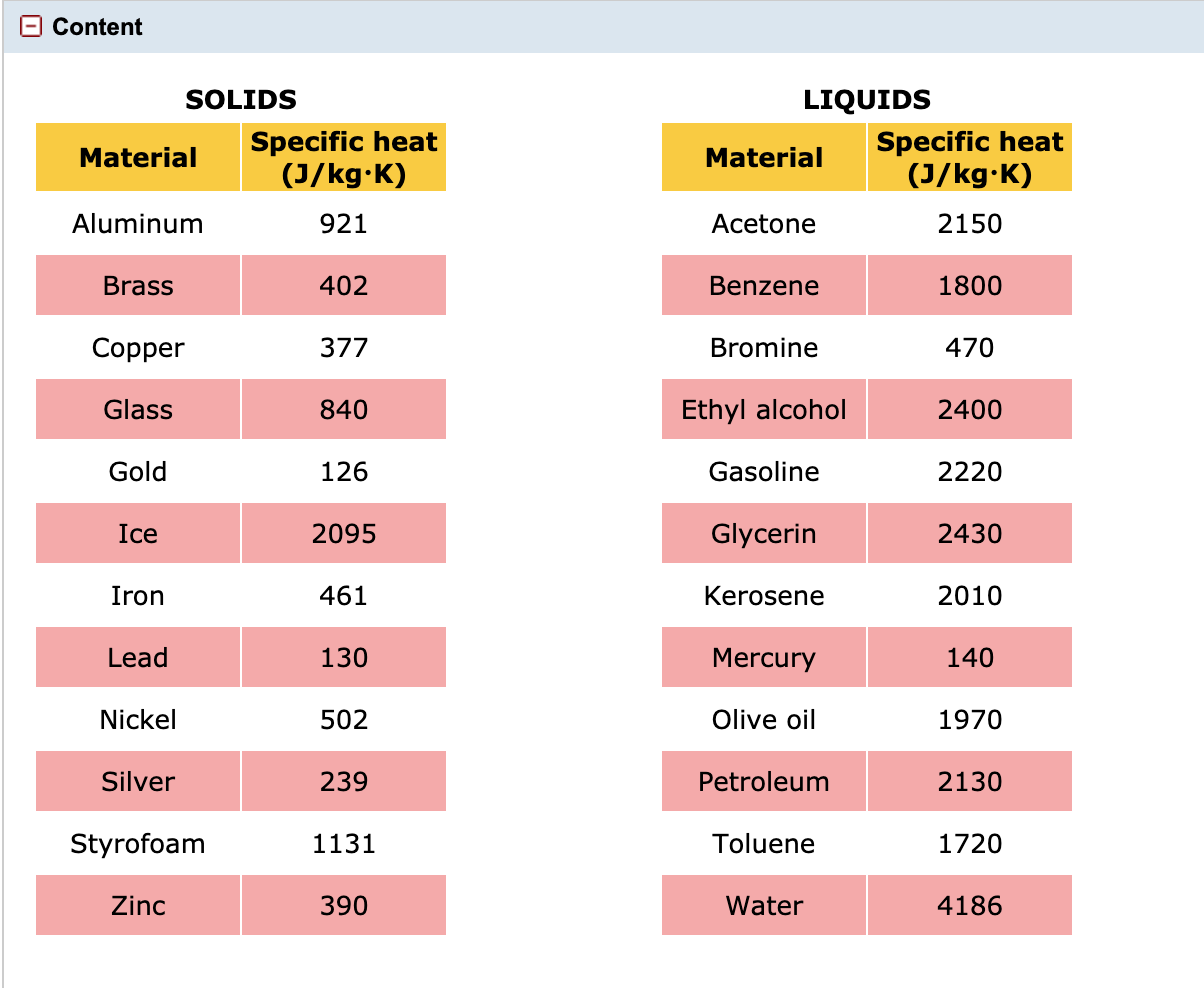
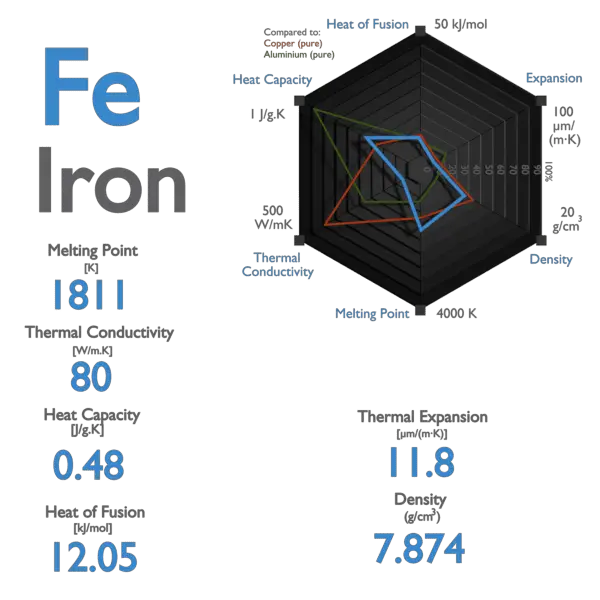
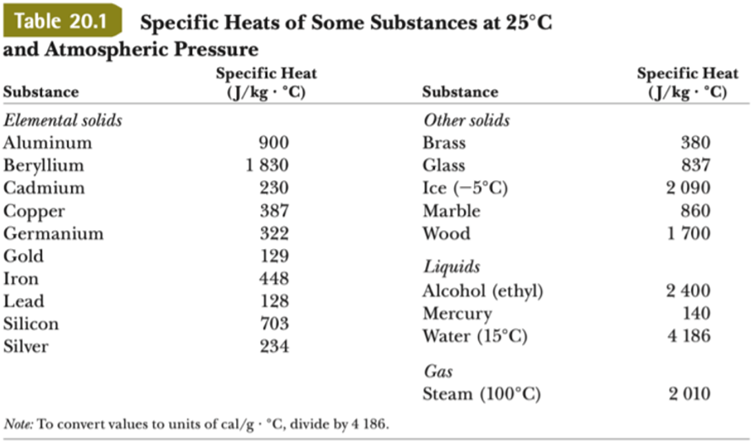
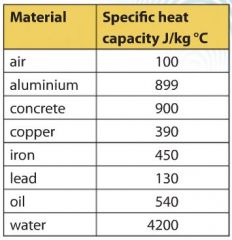
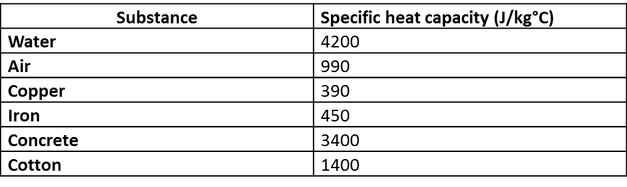
📐PSYW - Please Show Your Work A radiator made out of iron of specific heat capacity 450 J/kgk has a - Brainly.com

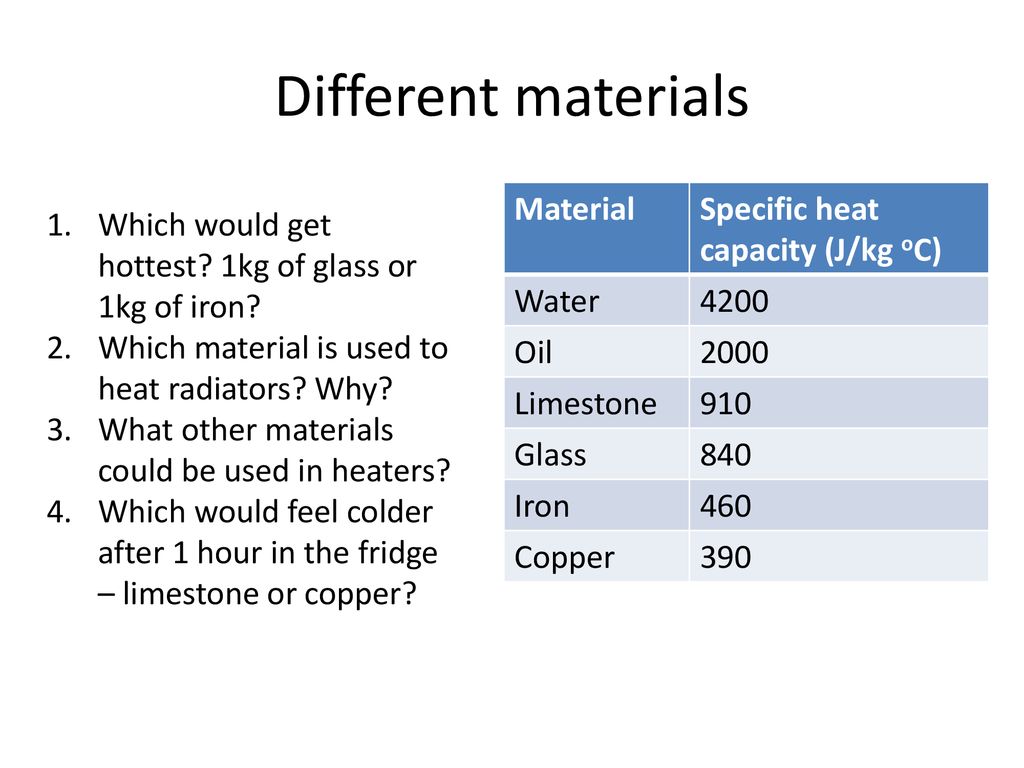
Learning Outcomes: Rearranging equation for Specific Heat Capacity Topic Equation for Specific Heat Capacity Target Audience: G & T Teacher instructions. - ppt download

250 g of water at 30^∘C is contained in a copper vessel of mass 50 g . Calculate the mass of ice required to bring down the temperature of the vessel and

1. Calculate the amount of heat required to change the temperature of an iron ball of mass 3 kg from 30° C - Brainly.in
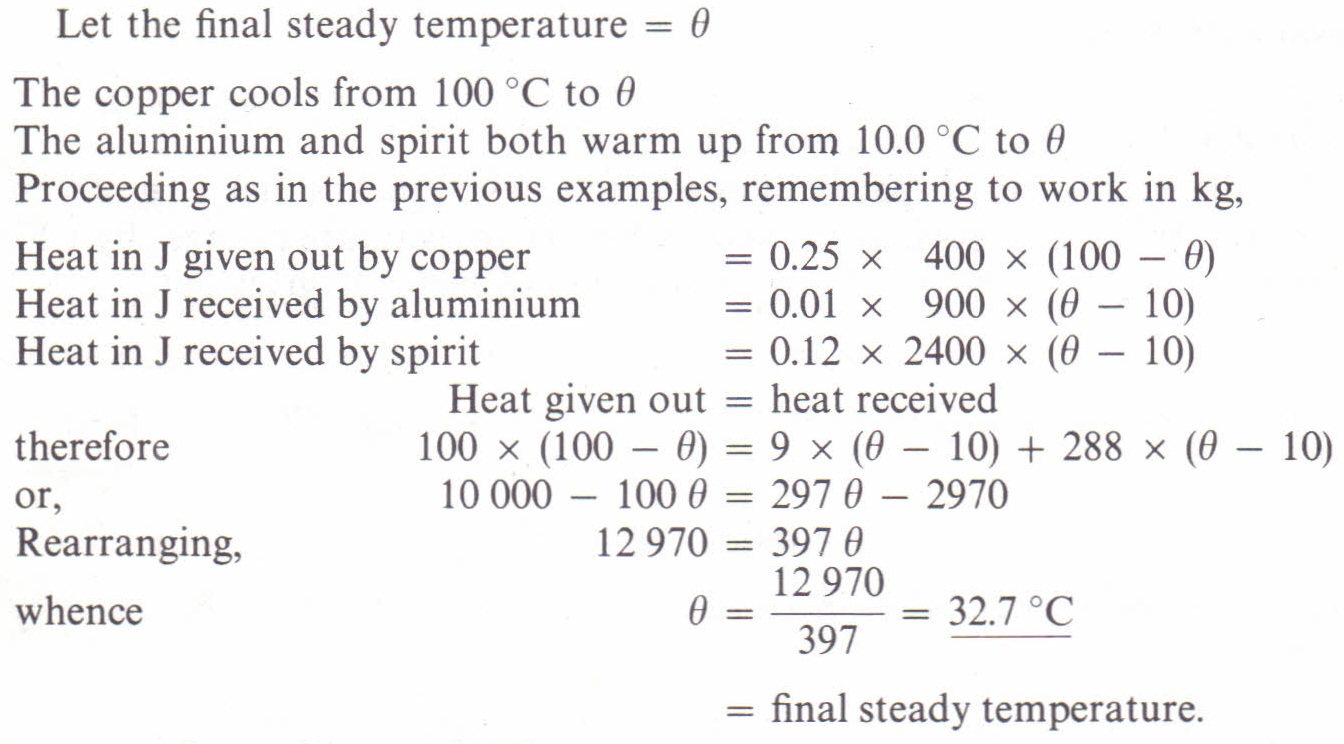
Cheat calculations Physics Homework Help, Physics Assignments and Projects Help, Assignments Tutors online
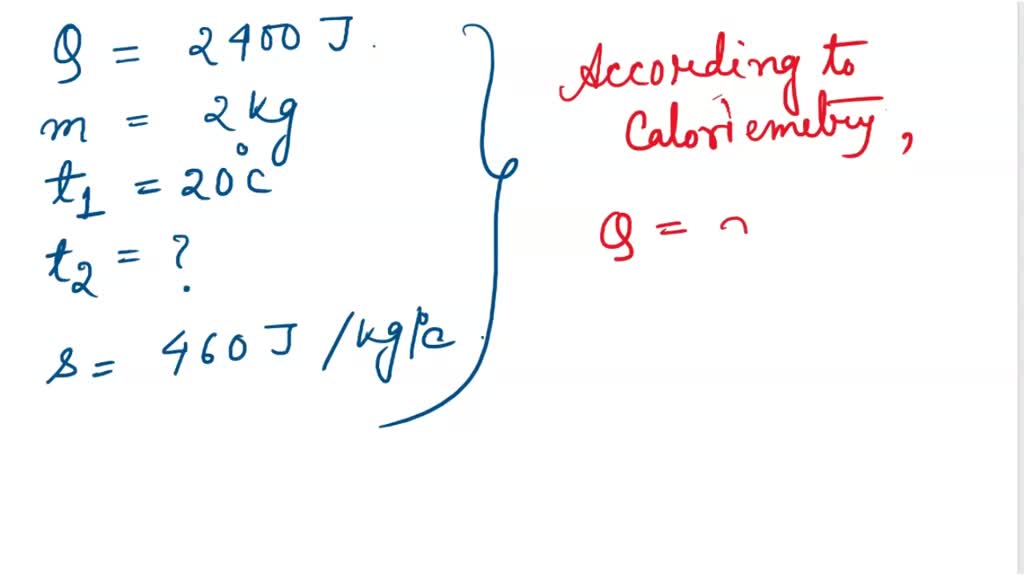
SOLVED: Calculate the final temperature when 2400 joules of heat is given to an iron of mass 2kg at 20°C. (Specific heat capcity = 460 J/kg° C)