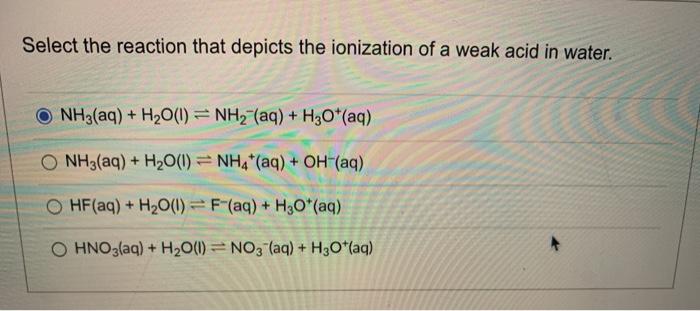
![Liquid ammonia ionizes to slight extent. At - 50^∘C , its self - ionization constant, K = [NH4^+] [NH2^-] = 10^-30 M^2 . How many amide ions are present per ml of pure liquid ammonia? ( NA = 6 × 10^23 ) Liquid ammonia ionizes to slight extent. At - 50^∘C , its self - ionization constant, K = [NH4^+] [NH2^-] = 10^-30 M^2 . How many amide ions are present per ml of pure liquid ammonia? ( NA = 6 × 10^23 )](https://dwes9vv9u0550.cloudfront.net/images/11548439/2313bf3e-ac46-4626-8ef0-7c7a53c9fba2.jpg)
Liquid ammonia ionizes to slight extent. At - 50^∘C , its self - ionization constant, K = [NH4^+] [NH2^-] = 10^-30 M^2 . How many amide ions are present per ml of pure liquid ammonia? ( NA = 6 × 10^23 )

Energy spectrum of the electrons emitted in the ionization of ammonia... | Download Scientific Diagram
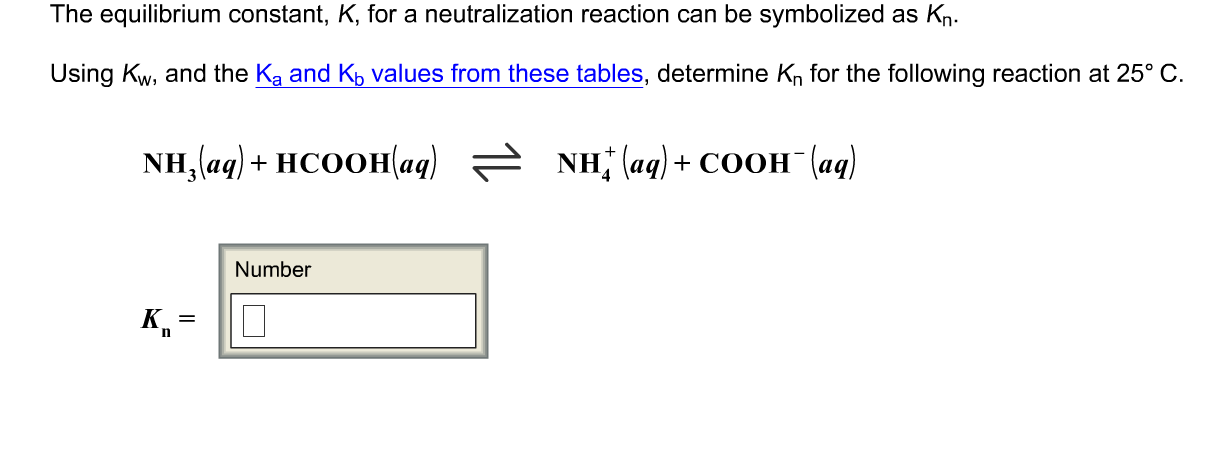
![Liquid ammonia ionizes to slight extent. At - 50^∘C , its self - ionization constant, K = [NH4^+] [NH2^-] = 10^-30 M^2 . How many amide ions are present per ml of pure liquid ammonia? ( NA = 6 × 10^23 ) Liquid ammonia ionizes to slight extent. At - 50^∘C , its self - ionization constant, K = [NH4^+] [NH2^-] = 10^-30 M^2 . How many amide ions are present per ml of pure liquid ammonia? ( NA = 6 × 10^23 )](https://haygot.s3.amazonaws.com/questions/1946954_1680835_ans_ea35f931269d4fc69c74338ac2033af8.jpg)
Liquid ammonia ionizes to slight extent. At - 50^∘C , its self - ionization constant, K = [NH4^+] [NH2^-] = 10^-30 M^2 . How many amide ions are present per ml of pure liquid ammonia? ( NA = 6 × 10^23 )
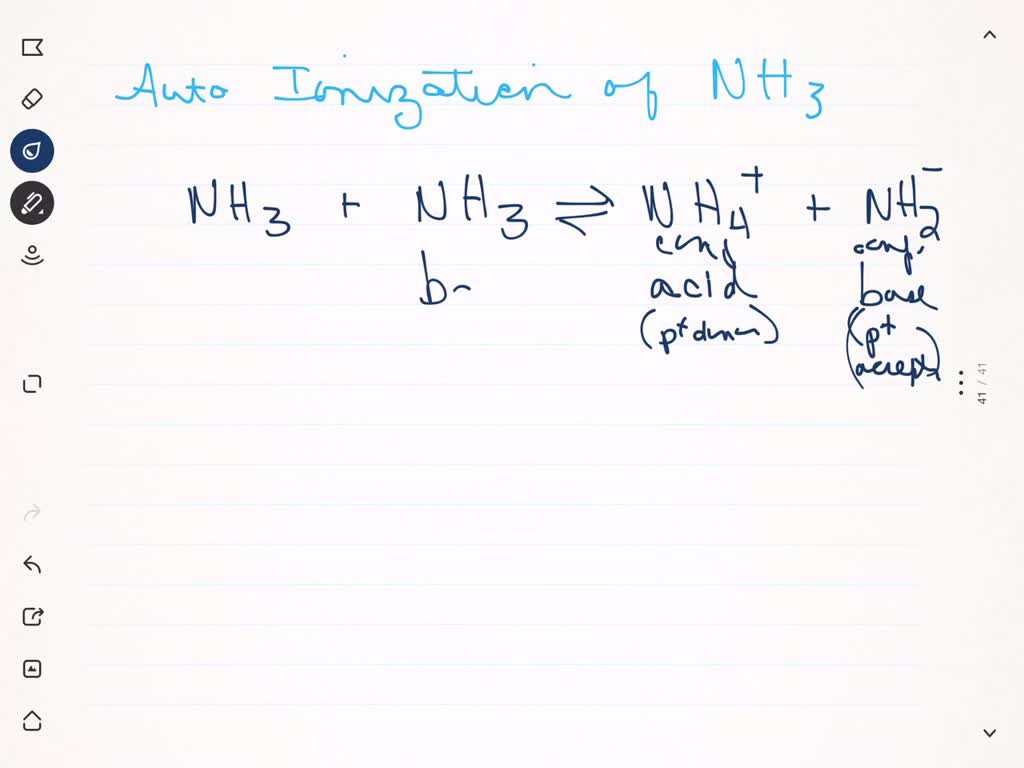
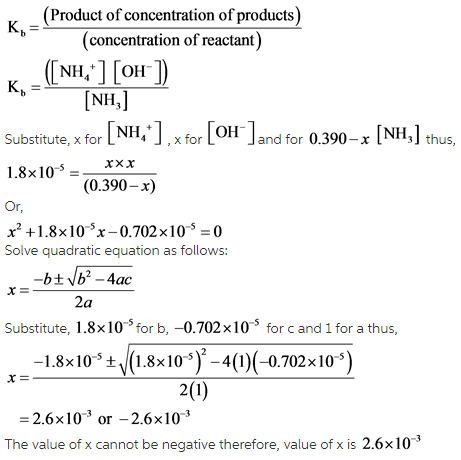
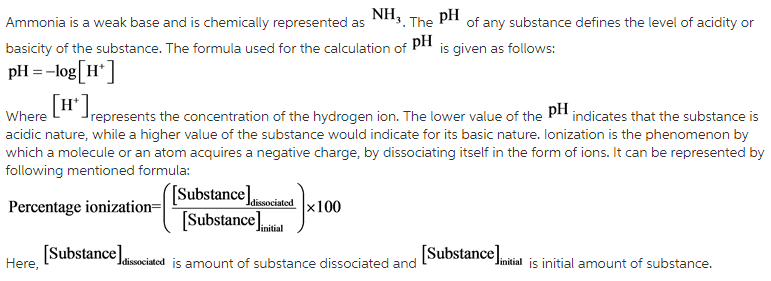
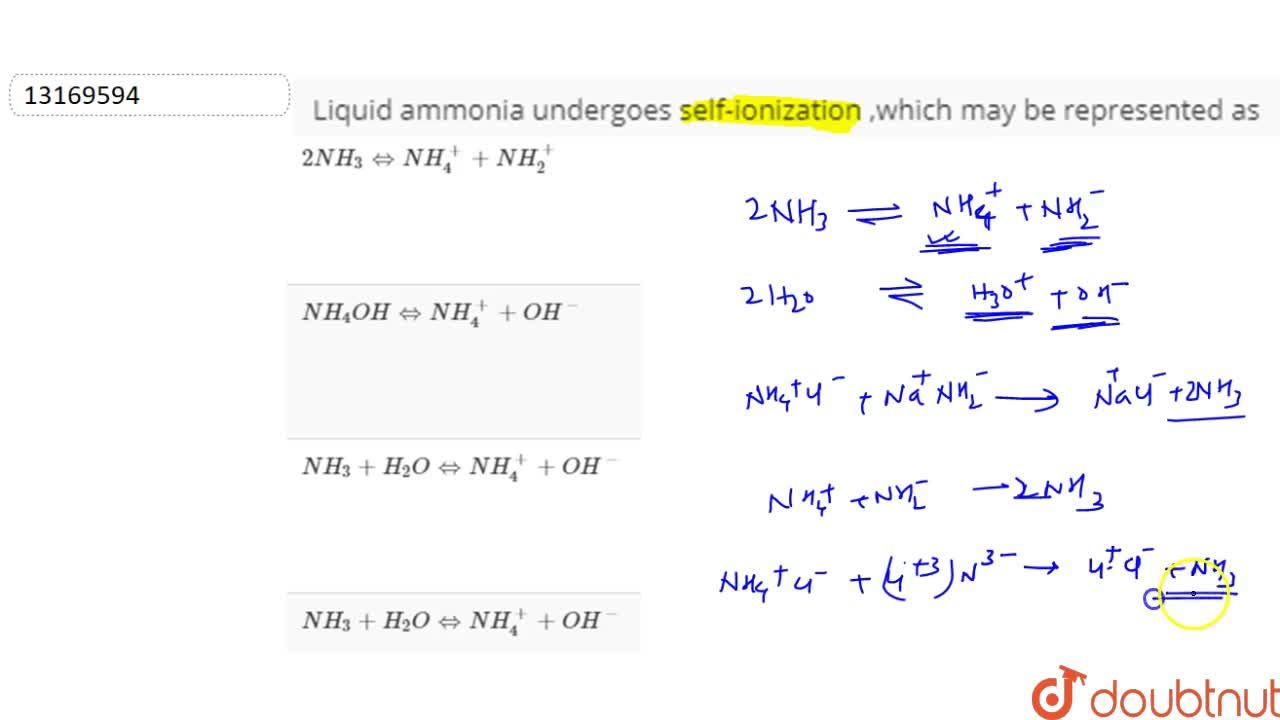
SOLVED:Like water, liquid ammonia undergoes autoionization: NH3+NH3 ⇌NH4^++NH2^- (a) Identify the Bronsted acids and Bronsted bases in this reaction. (b) What species correspond to H^+ and OH^- and what is the condition
![At - 50^(@) C, the self - ionization constant (ion product ) of NH(3)" is " K(NH(3)) = [NH(4)^(+)] [NH(2)^(-)] = 10^(-30) M^(2). How many amide ions are present per mm^(3) of pure liquid ammonia ? At - 50^(@) C, the self - ionization constant (ion product ) of NH(3)" is " K(NH(3)) = [NH(4)^(+)] [NH(2)^(-)] = 10^(-30) M^(2). How many amide ions are present per mm^(3) of pure liquid ammonia ?](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/261016062_web.png)
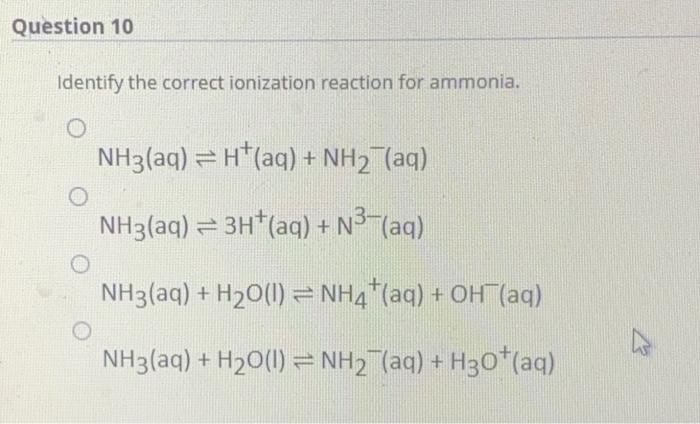
.jpg)



![Give evidence that [ Co NH 35 Cl ] SO 4 and [ Co NH 35 SO 4] Cl are ionization isomers. Give evidence that [ Co NH 35 Cl ] SO 4 and [ Co NH 35 SO 4] Cl are ionization isomers.](https://search-static.byjusweb.com/question-images/img/study_content/curr/1/12/17/268/6722/NCERT_19-11-08_Sonali_12_Chemistry_9_11_GSX_html_m25d3536d.gif)