The General Court enhances legal protection for producers of generic pharmaceuticals by allowing an incidental plea of illegality against a Commission decision authorising the marketing of a reference medicine | & DE

Brexit: End of Mutual Recognition with the UK - European Directorate for the Quality of Medicines & HealthCare

Good Manufacturing Practice (Gmp) Guidelines: The Rules Governing Medicinal Products in the European Union, Eudralex Volume 4 Concise Reference a book by Mindy J. Allport-Settle
Marketing authorisations which are recommended for maintenance and marketing authorisation applications for which bioequivalence

Book 4C: 2022 Good Manufacturing Practice in the European Union, Refer – Clinical Research Resources, LLC
ad hoc working group on validation issues/national requirements common grounds for invalidation/delaying validation
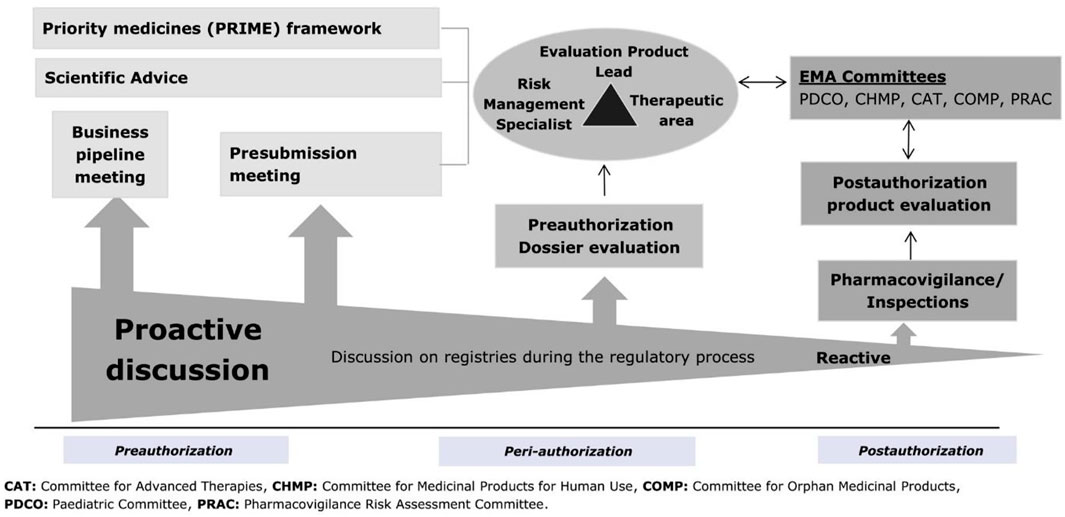
Frontiers | Contribution of patient registries to regulatory decision making on rare diseases medicinal products in Europe
CMD(h) WORKING DOCUMENT - INFORMATION TO BE SUBMITTED BY THE MEMBER STATE OF THE EUROPEAN REFERENCE MEDICINAL PRODUCT January 20

Marketing authorization and licensing of medicinal products in EU: Regulatory aspects - ScienceDirect