2 mole of ideal gas at 27°C temperature is expanded reversibly from 2 lit. to 20 lit. - Sarthaks eConnect | Largest Online Education Community
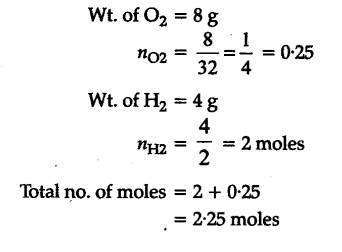
Calculate the total pressure in a mixture of 8g of dioxygen and 4 g of dihydrogen confined in a vessel of 1 d${{m}^{3}}$ at 27°C. R = 0.083 bar d${{m}^{3}}$${{K}^{-1}}$${{mol}^{-1}}$ - CBSE

Calculate the total pressure in a mixture of 8 g of dioxygen and 4 g of dihydrogen confined in a vessel of 1 dm ^3 at 27 ^∘C . (R = 0.083 bar dm ^3 K ^-1 mol ^-1 )
An ideal gas heat engine operate between 27°c and 127°c if it absorbs 10 kcal per cycle at higher temperature then amount of heat energy thrown to sink per cycle is
For two reactions in equilibrium in same container() A(s)2B (g) + C(g); (K,), = 27 atm3(ii)D(s)2B(g) +E(g); (Kp)= 81 atm3Pressure of B(g) at equilibrium will be(1) 6 atm(2)3 atm(3) 5 atm(4) 9



:max_bytes(150000):strip_icc()/TC_609228-convert-celcius-to-fahrenheit-5ab27b90c064710036ce2d0a.gif)